| |
|
|
The conclusions of this paper are like this :
NIR for fermentation and lyophilisation processes
Fermentation and cell culture applications
- NIR useful at multiple stages of fermentation-related processes
- Upstream: raw materials qualification
- In-process: real-time monitoring of matrix condition and product titer
- Downstream:
• Qualitative analysis of column material + real time monitoring of the purification process
• Real-time monitoring of modification process
- Key aspects:
• Proper preliminary matrix categorisation
• Increasing availability of papers related to manufacturing implementations
Lyophilisation
- Technology potential: achieve true QbD as alternative to QC testing
- Main challenge: in-line analyser interfacing to freeze-dryer
- Expect that first advances in
manufacturing will be in the field
of process monitoring
rather than control
|
|
|
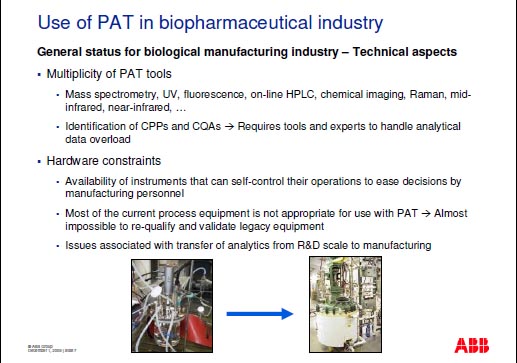
Generell Aspects in the Biomanufacturing Industry
The biomanufacturing industry still shows some resistance to the implementation of process NIR technology :
| only 3% have implemented PAT initiatives |
Three hurdles can mainly be classified :
- Regulatory absence : biotech companies have been slow to embark on the Process Analytical Technology (PAT) and Quality by Design (QbD) journey in particular due to the absence of a guidance by FDA on the use of PAT for biologics.
- The economic relevance of analytical in-line implementation is not always easy to justify.
- Technological : The applicability of NIR technology is largely dependent on the complexity of the matrix.
Focus on the Technological Challenges
This lecture places a special focus on the technological challenges.
When moving from the laboratory stage into manufacturing special attention must be paid to scale-up effects and heterogeneous process data management. |
|
Use of PAT in biopharmaceutical industry |
|
Use of PAT in biopharmaceutical industry |
The complexity of biologicals
- Proteins can be extremely heterogeneous and have complex structure
- Characterization not easy
- Fermentation media contain multiple ingredients and possible interferents
- Variability of raw materials and seed inoculum
- Broad dynamic range in product titers (mg/l to g/l)
- Biological products are heat sensitive and susceptible to microbial contamination
- Need for aseptic equipments and principles from initial manufacturing steps in contrast
to most conventional drugs.
- Culture yield very sensitive to process parameters variations (temperature, aeration,
agitation)
|
|
|
NIR for fermentation
and cell culture
applications
|
|
NIR for fermentation
and cell culture
applications |
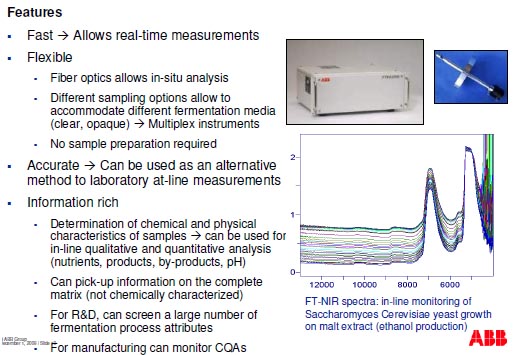
Monitoring of the fermentation process
- Cell density and biomass content
- Example: determination of biomass growth by correlation of FT-NIR spectra with cell
density during very-high-gravity corn mash fermentation (NCSU-BTEC)
- Determination of fermentation analytes concentration (nutrients, metabolites, byproducts)
- Example: monitoring of total sugars and ethanol from FT-NIR spectra during very-highgravity
corn mash fermentation (NCSU-BTEC)
|
|
 |
NIR for fermentation
and cell culture
applications |
|
NIR for fermentation
and cell culture
applications |
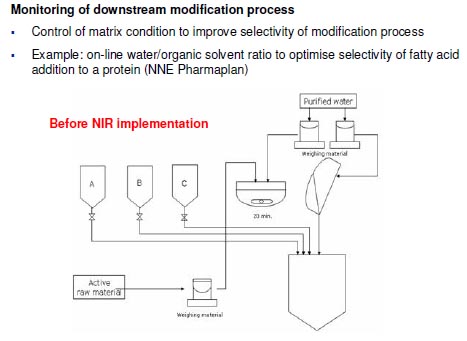
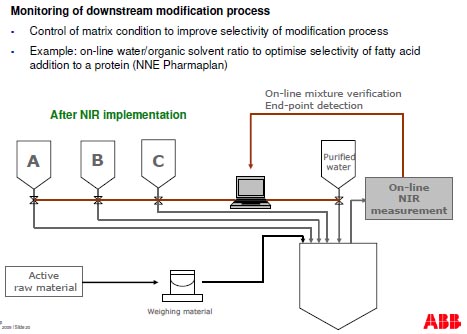
Monitoring of downstream purification process
- Monitor the elution process and fraction collection to reduce loss of API through ionic
exchange column (alternative to assay that takes 10 minutes
- Example: Use of FT-NIR to control chromatography column gradient mixing in a
protein purification process (NNE Pharmaplan)
|
|
 |
| NIR for fermentation
and cell culture
applications |
|
NIR for fermentation
and cell culture
applications |
Data evaluation and Chemometrics
- Spectral region optimisation (exclude C-H combination region below
4800 cm-1 and strong water band at 5100 cm-1)
- Full spectra algorithms preferred to extract complex matrix information
- Incorporate temperature fluctuations in model
- Consider time-segmented calibrations to handle fast matrix changes
- Use of spectral preprocessing
- Effect of agitation rate, gas flow rates and biomass variations on scattered
light variations
- Baseline shifts
- Use process trajectories to detect culture contaminations
- Use on-the-fly statistical evaluation of model applicability to detect
extrapolation conditions where model robustness can be challenged
(e.g. F-ratios)
|
|
 |
| NIR for
lyophilisation
applications |
|
NIR for
lyophilisation
applications |
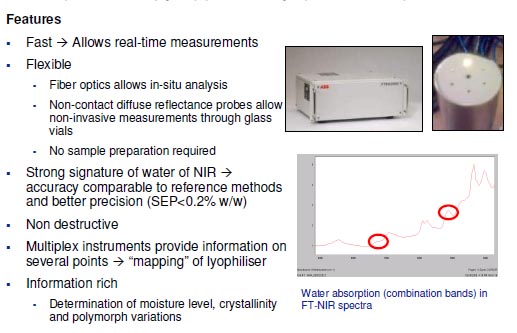
PAT sensors for lyophilisation processes
Objectives for lyophilisation
- Real-time monitoring of moisture content for end-point determination of freeze-drying process
- � Achieve real time feedback control to achieve “right first rime” quality
At-line methods
- Loss on drying (IR)
 lack of repeatability, destructive, can not distinguish free/bonded water lack of repeatability, destructive, can not distinguish free/bonded water
- Karl-Fischer titration
 destructive, slow, requires skilled operators destructive, slow, requires skilled operators
Some PAT sensors
- Temperature / conductivity probes inserted in vials :
• Potential crystallisation points during freeze-drying
• Sterility issues
• Non-representative information
- Mass spectroscopy (Headspace measurement on vaporised water) :
• No process interference
• Provides overall process end-point information
• No direct measurement of CQA
• Requires constant leak rate  frequent leak tests required frequent leak tests required
|
|
 |
| NIR for
lyophilisation
applications |
|
NIR for
lyophilisation
applications |
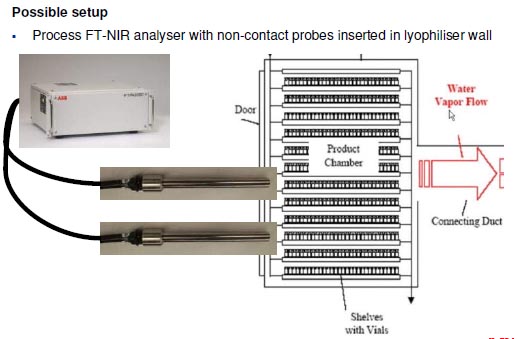
Technical challenges – Probe-related aspects
- Availability of sampling points
• Not possible to retrofit legacy equipment
• Collaboration with freeze dryer supplier required early in project
• Ideally have sampling points coupled with windows
- Positioning of sampling points
• Usually only external vials accessible due to tray design with silicon oil circulation
between shelves
Monitoring only (cycle-time reduction)  OK OK
Feedback control or parametric release  Requires careful validation to demonstrate representativity of measurements (drying usually not homogeneous) Requires careful validation to demonstrate representativity of measurements (drying usually not homogeneous)
• Reproducibility of probe alignment in front of vial can be challenging (automated tray
loading, possibly different vial diameters depending on products)  Possibility to
visually preposition retractable non-contact probe after tray loading and manually fine
tune probe positioning by using XYZ 3-D stage and maximising reflectance signal after Possibility to
visually preposition retractable non-contact probe after tray loading and manually fine
tune probe positioning by using XYZ 3-D stage and maximising reflectance signal after
water sublimated (powder), or early in process by focusing beam on stopper.
-
Specifications
• Probe with aseptic design that sustain P and T conditions, including SIP cycle
temperature gradient
• Vacuum-tight flange seal
|
|
 |
| Zusätzliche Informationen : |
|
|
 Download Vortrag Download Vortrag
|
|
|
| |
  |







